General Information
- An osteosarcoma is so called, because it is a cancerous tumor that is derived from a mesenchymal stem cell precursor (thus, by definition a sarcoma). The malignant cells produce immature woven bone, or osteoid, which is why the tumor is named osteosarcoma--it is a bone-producing sarcoma.
- Osteosarcomas most commonly arise from bones although they can also rarely arise from soft tissues of the extremities (muscles and connective tissues of the extremities).
- Microscopically, the tumor cells are called spindle cells, as they are cigar-shaped.
- There are many types of osteosarcomas. The most common is called conventional osteosarcoma.
- In general, osteosarcomas can be classified into three general categories according to where they arise from the bone:
- Intramedullary: Most common; arise from middle of bone (medullary canal)
- Surface/Juxtacortical: Arise from the surface or periosteum of the bone
- Intracortical: Extremely rare; arise from within the cortex of the bone
| Subtypes of Osteosarcoma |
Intramedullary (75%)
- Conventional
- Osteoblastic (82%)
- Mixed and Sclerosing
- Chondroblastic (5%)
- Fibroblastic (3-4%)
- MFH-like (3-4%)
- Osteoblastoma-like (.5%)
- Giant Cell-rich (.5%)
- Small-cell (1%)
- Epithelioid (.5%)
- Telangiectatic (3%)
|
Juxtacortical/Surface (7-10%)
- Parosteal
- Periosteal
- High-grade surface
- Intracortical (.2%)
Secondary (older population)
- Pagets (67-90%); Post RT (6-22%); Bone infarct; Fibrous dysplasia; Metallic implant; Osteomyelitis
Osteosarcoma associated with specific syndromes
- Familial; Retinoblastoma; Rothmund-Thomson Syndrome; Multifocal; OI
|
- Osteosarcoma is the second most common primary malignant tumor of bone
- It is the most common primary cancer of bone in children and adolescents
- Osteosarcomas represent 15% of all biopsied primary bone tumors
- There are approximately 600 to 700 new cases of osteosarcoma in the United States per year
- Osteosarcomas are more common in children than adults
- The most common type of osteosarcoma is the Primary, High Grade, Intramedullary (Conventional) Osteosarcoma, which represents approximately 75% of all osteosarcomas
Definitions:
- Primary Osteosarcoma: arises from the bone in the absence of a precursor lesion or treatment
- Secondary Osteosarcoma: arises from a precursor lesion or one that is metastatic from a primary osteosarcoma
- Synchronous Osteosarcoma: Lesions that affect multiple bones discovered within 6 months of each other
- Metachronous Osteosarcoma: Lesions involving multiple bones discovered more than 6 months apart
Clinical Presentation
Signs/Symptoms associated with an osteosarcoma:
- Mild Pain for weeks-months
- Pain gradually becomes more severe & accompanied by swelling and limitation of motion
- Weight loss is correlated with disseminated disease
- Blood tests may demonstrate a high serum alkaline phosphatase
Prevalence:
- Slightly more common in males than females (ratio:1.5-2:1) possibly due to longer period of male skeletal growth
Age of developing an osteosarcoma:
- Two peak age groups (rare <6y or >60y)
- First peak: 15-25 years: Most common age is in childhood and adolescence
- Second peak (much smaller) – just over 50 years: Less common in adults
- When osteosarcomas occur in adults, there is often an underlying predisposing condition such as a history of radiation to the bone, Paget's Disease, or an underlying bone infarct
- When an osteosarcoma arises from a pre-existing condition of bone, it is called a secondary osteosarcoma
Anatomic sites of developing an osteosarcoma:
- Long Bones (Most common: 70%-80% of cases)
- Most (90%) arise from the metaphysis of the bone; 10% from the diaphysis
- Thus in most instances, the tumor arises next to a joint
- Distal Femur: most common site (40%; about twice as common as proximal tibia)
- Proximal Tibia: Second most common site
- Proximal Humerus: Third most common site
- Axial Skeleton: Pelvis, Spine are much less common sites. Most patients present with grossly detectable (detectable with radiology studies) disease isolated to the extremity. This means that with conventional X-rays, CT scans, MRIs the tumor can only be detected in the extremity (leg, arm, pelvis or spine)
The most common sites for developing metastases are:
- Lungs: Most common site
- CT of the chest is used for detecting lung metastases
- Bones: Second most common site
- whole body bone scan is used for detecting bone metastases
- Liver: Rare site
- Approximately 15%-20% of patients present with detectable metastases to the lungs. Fewer patients present with bone metastases
- Most patients do not develop bone metastases without developing lung metastases first. Rarely, however, bone metastases occur without lung metastases.
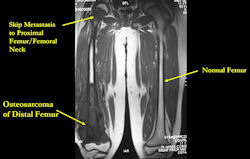
Skip metastases: metastases within the same bone, or across the joint in the adjacent bone, as the primary osteosarcoma
- spread via intraosseous or transarticular venous system within the bone
- rare; may occur without any evidence of pulmonary metastases
- traditionally associated with poor prognosis, although recent reports may suggest otherwise
- detection of skip metastases is important for surgical planning; occasionally, presence of skip metastases may dictate that the entire bone be surgically removed
| Roll over the images for more information |
 |
Historical Treatment Of Osteosarcoma
- before chemotherapy developed, patients with osteosarcoma isolated to the extremity (no evidence of lung metastases on chest X-rays) treated with an amputation
- despite amputation, 83% of patients still developed pulmonary (lung) metastases and died of disease
- only 17% of patients were cured with amputation: had very small tumors that were detected early
- with no detectable lung disease at presentation, patients had microscopic lung disease that ultimately grew and resulted in death
- amputation removed disease in the extremity, but did not address the microscopic disease
- chemotherapy developed to treat microscopic disease
- when patients with isolated high grade osteosarcomas were treated with chemotherapy and surgery, micrometastatic (microscopic) disease was eradicated, and most patients capable of being cured
- surgeons discovered they were able to also save extremities (arms and legs) of patients with high grade osteosarcomas, especially when chemotherapy administered before surgery (hence the term limb-sparing surgery)
- preoperative (also called neoadjuvant) chemotherapy kills the main tumor, making limb sparing surgery possible
- chemotherapy also kills microscopic disease in the lungs
- tumors were studied after removal from patients after treatment with chemotherapy: patients with good response (approximately 90% tumor cell death) to preoperative chemotherapy had a better prognosis or chance for cure than patients who did not have a good response
- all patients receiving preoperative chemotherapy had better prognosis than if they had not received chemotherapy at all, regardless of response
- definition of "good response" to preoperative chemotherapy is controversial and varies amongst centers: some believe 90% tumor necrosis confers a better prognosis (85%-90% cure rate), while others use 99% tumor necrosis to predict a better prognosis
Radiology
(see below for detailed information on radiographic presentation of osteosarcomas)
Xray (plain radiographs) of the extremity
MRI with contrast (gadolinium) of the extremity
Best for:
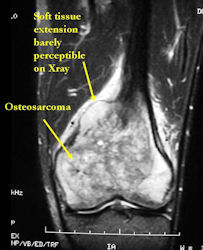
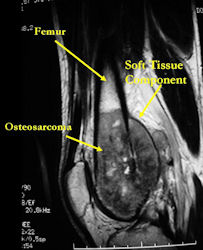
- determining intraosseous extent (size) of the tumor and size of the soft tissue component
- determining relationship of tumor to neurovascular structures
- detecting skip metastases
- Entire bone and adjacent joint should be visualized
- Not good for determining response to preoperative chemotherapy
CT (CAT) Scan of the tumor/extremity
- Complementary to MRI
- Useful for:
- detecting subtle tumor calcification and ossification diagnostic of an osteosarcoma
- evaluating cortical bone integrity and penetration beyond the cortex
- evaluating tumor extent when there is extreme edema on the MRI
- evaluating response to preoperative chemotherapy (pre-chemo CT compared to post chemo CT before surgery)
CT Scan of the Chest: Used to detect pulmonary metastases
Whole Body Bone Scan: Used to detect bone metastases, extent of bony involvement by the tumor and presence of skip metastases
- should be correlated with the MRI and CT scan
Thallium Scan: special type of bone scan useful for evaluating response to preoperative chemotherapy
- Not routinely ordered in all centers
CT PET Scan of Whole Body: Not yet demonstrated to be reliable for detecting metastases nor for evaluating response to preoperative chemotherapy before surgery
Angiography: Not routinely performed; indicated in selected circumstances
- contrast injected directly into main artery of the extremity, then x-rays taken of the blood vessels around the tumor
- gold standard for evaluating response to preoperative chemotherapy
- good response to preoperative chemotherapy: no significant uptake of contrast dye; viable tumors: show vascular tumor blush, because blood supply to tumor maintained, and fills with dye
- also useful for preoperative planning: showing how the blood vessels are displaced or moved by the tumor, determining proximity of blood vessels to tumor
Treatment General Information
(see below for more detailed information about treatment and surgery)
- almost all osteosarcomas are high grade tumors (grow quickly and have a high likelihood of spreading/metastasizing)
- exceptions: well differentiated intraosseous osteosarcomas and parosteal osteosarcomas--both are low grade tumors (these grow slowly and rarely spread/metastasize)
- all high grade osteosarcomas (including conventional osteosarcoma) treated with a combination of chemotherapy and surgery.
- usually, chemotherapy is administered before and after surgery
- chemotherapy before surgery = preoperative = neoadjuvant chemotherapy
- chemotherapy after surgery = adjuvant chemotherapy
- radiation therapy rarely recommended for osteosarcomas
- mostly in pelvic and spine cases, when unable to obtain a wide surgical margin
- decision to administer radiation depends on the size of the tumor and response to preoperative chemotherapy
- Low grade osteosarcomas usually treated with surgery alone (parosteal osteosarcoma; well differentiated intramedullary osteosarcoma)
- can sometimes dedifferentiate, or develop high grade areas, when present for prolonged periods of time
- dedifferentiation may not be detected until entire tumor removed; chemotherapy may be indicated after surgery
Periosteal osteosarcomas are intermediate grade tumors
- treatment usually similar to conventional osteosarcoma
Surgery
- Most patients can be treated with limb sparing surgery and reconstruction, instead of an amputation
Much of the success of limb-sparing surgery is due to several factors:
- Preoperative chemotherapy induces tumor necrosis, enables the body to initiate a healing response: edema (swelling) decreases; less soft tissue needs to be removed with the tumor; intraoperative determination of normal vs. abnormal tissue is easier; tumor size may not shrink because of osteoid and bone, but malignant cells may be killed
- Advanced radiological imaging modalities (MRI and CT) help determine the exact extent of the tumor and involvement of neurovascular structures before surgery
- Advances in prosthetic design and surgical technique
Limb sparing surgery for osteosarcoma of bone is usually a wide or radical resection
- after resection, bone and/or joint and soft tissues reconstructed:
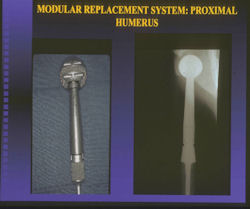
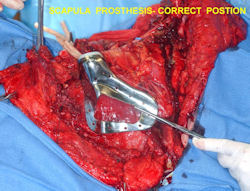
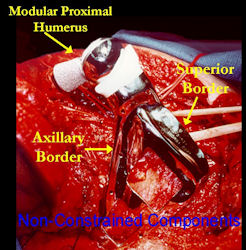
- one of the more favorable and common methods is a prosthetic reconstruction with a metallic, modular, segmental tumor prosthesis. Osteosarcomas most frequently occur next to joints; therefore, neighboring joint often requires resection and reconstruction
- allografts, free vascularized fibula transfers, bone grafts have also been used for selected cases
Contraindications for limb sparing surgery for osteosarcomas include:
- Inappropriately performed biopsy that significantly contaminates surrounding soft tissues (#1 cause for requiring an amputation)
- Infected osteosarcoma
- Direct invasion or encasement of important neurovascular structures (relative contraindication)
- occasionally, blood vessels can be removed with the tumor and reconstructed with a graft; there are high complication rates with this procedure and many patients ultimately lose the extremity
- occasionally, a nerve may need to be resected with the tumor and the patient can still have a functional extremity
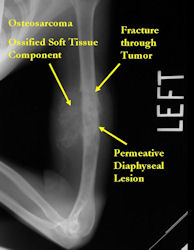
- pathological fracture (Relative contraindication)
- If pathological fracture can be stabilized nonoperatively and heals with preoperative chemotherapy, limb sparing surgery may be possible without compromising survival, as long as the contaminated tissue from fracture hematoma can be removed
- An extremely large tumor where resection with a wide margin would result in a useless extremity
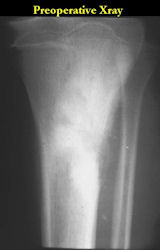
Radiographic Presentation: Conventional Osteosarcoma
There are 3 radiographic presentations for osteosarcomas, depending upon the amount of osteoid/ossification and calcium deposition:
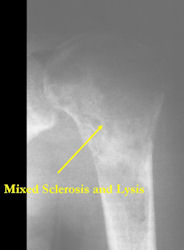
- Mixed sclerotic and lytic, permeative lesion most common radiographic presentation
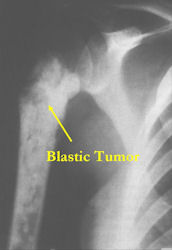
- Purely osteoblastic, permeative lesion: dense sclerosis and osteoid production
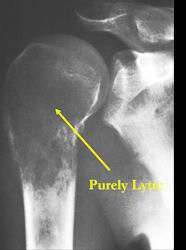
- Purely lytic, permeative lesion: little osteoid production and/or minimal calcium deposition in osteoid
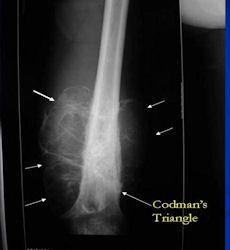
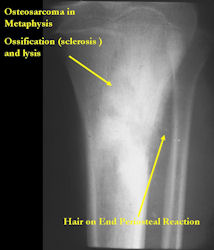
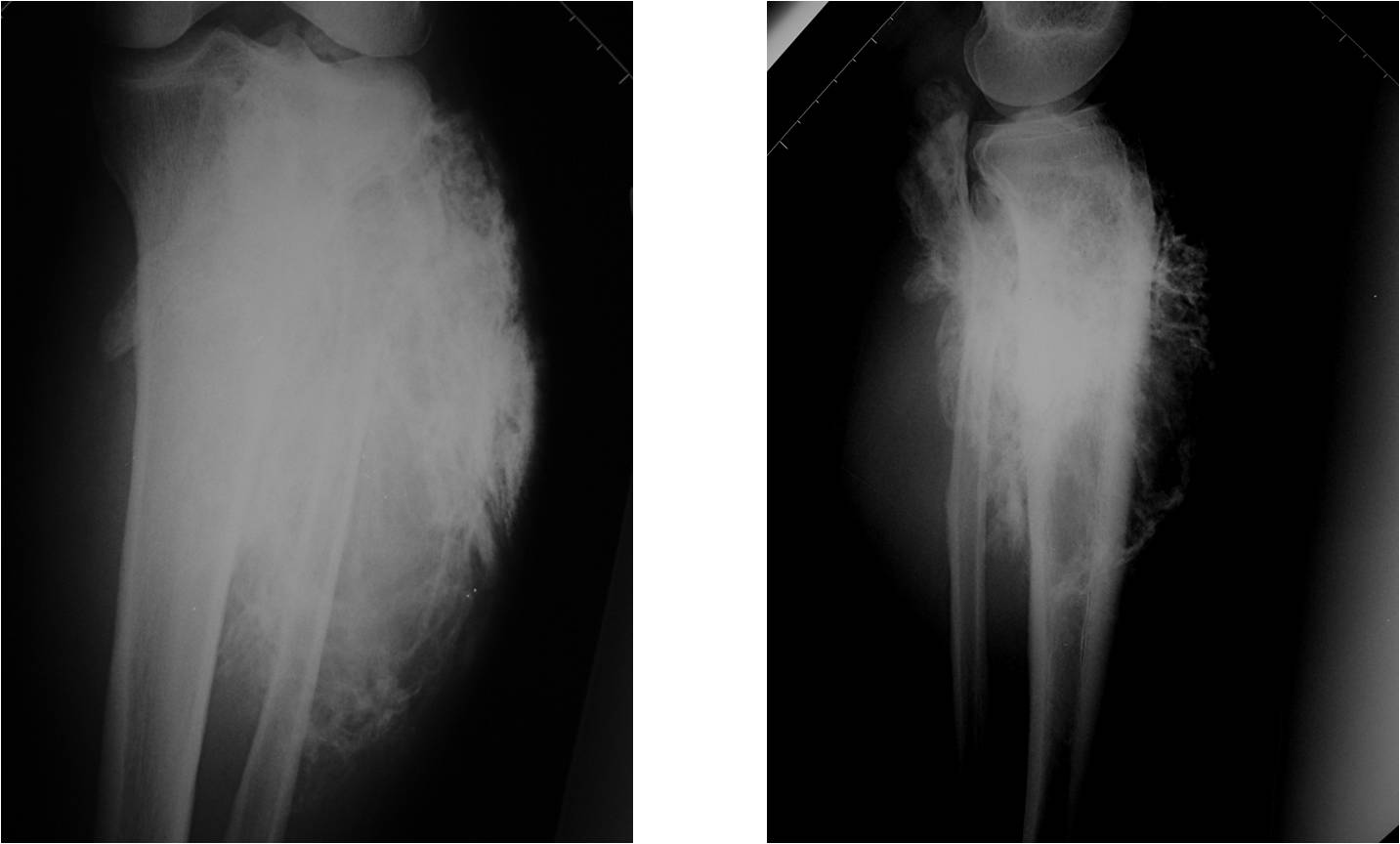
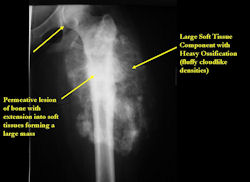
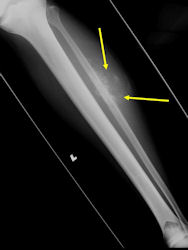
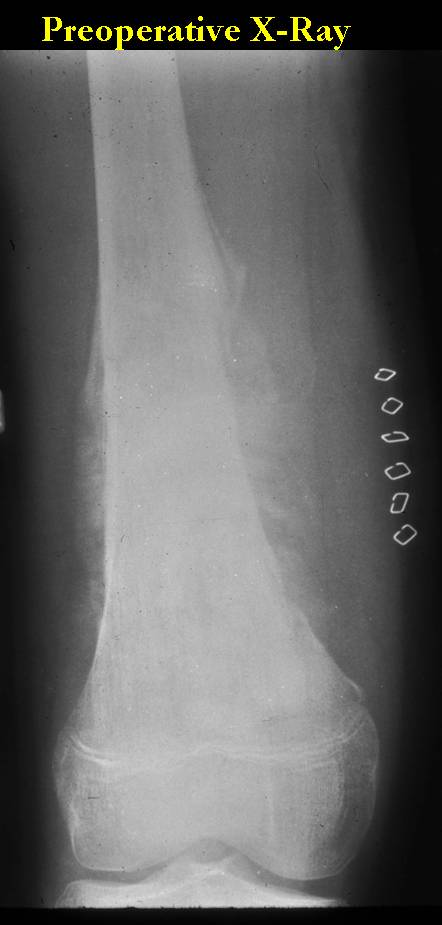
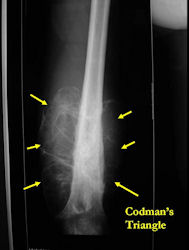
Conventional osteosarcomas are permeative lesions on plain radiographs (borders of the lesion cannot be clearly delineated)
- Wide zone of transition from lytic/sclerotic areas of tumor to normal bone
- Makes borders of lesion hard to define
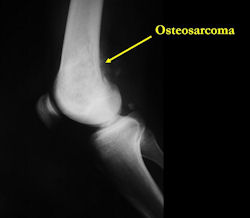
- Most (90%) arise from the metaphysis of the bone
- Rarely (10%) arise from the diaphysis
- Most conventional osteosarcomas (90-95%) extend through the bone into the soft tissues and form a soft tissue mass outside of the bone
| Roll over the images for more information |
 |
 |
 |
 |
 |
| Roll over the images for more information |
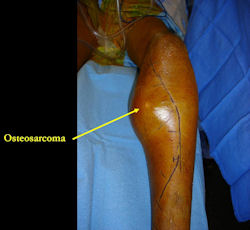
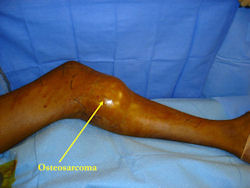
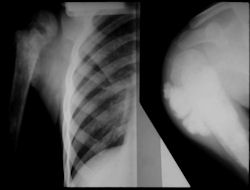
X-RAY: AP and lateral x-ray, osteosarcoma of distal femur
|
 |
 |
MRI of osteosarcoma of distal femur (same patient)
|
 |
 |
| Roll over the images for more information |
 |
 |
 |
 |
 |
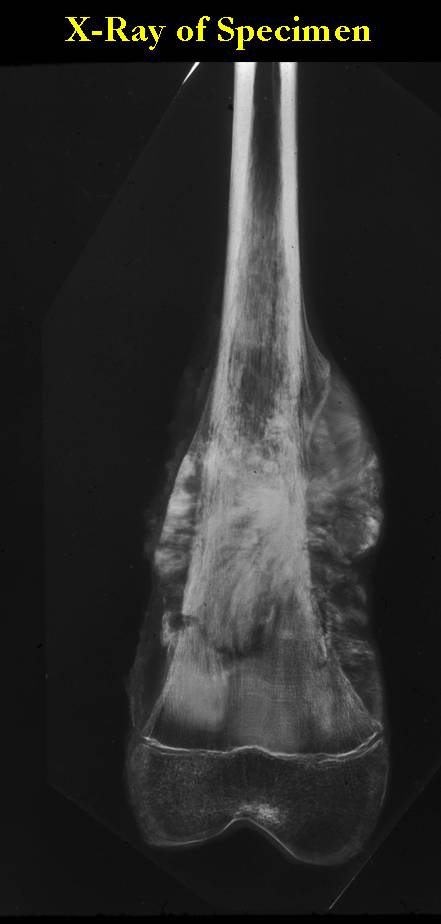
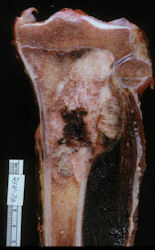
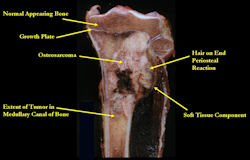
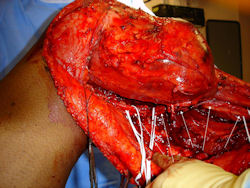
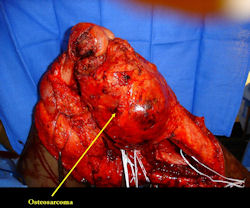
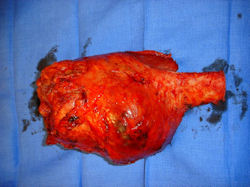
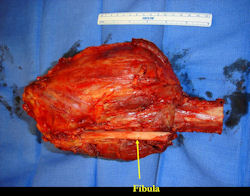
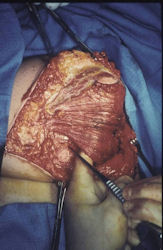
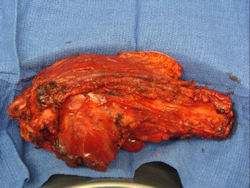
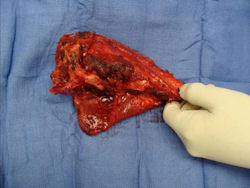
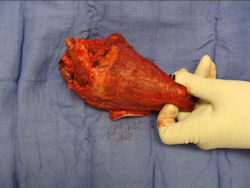
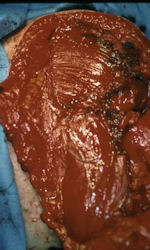
Gross Pathology: Conventional Osteosarcoma
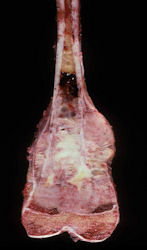
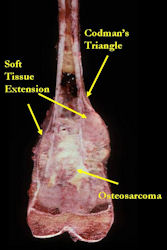
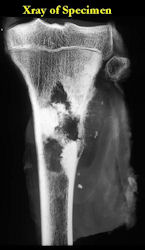
These are examples of the gross pathology specimens of resected conventional osteosarcoma, consisting of both bony and soft tissue areas. X-rays of the specimens are included.
- Osteosarcomas are composed of ossified or non-ossified tissue
- Ossified tissue is yellow-white and hard
- Less ossified tissue is soft and less yellow
- Non-ossified tissue is tan and fleshy
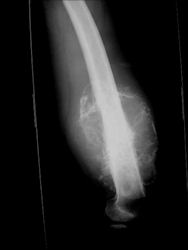
- Most (95%) of conventional osteosarcomas penetrate the cortex and form a large extraosseous soft tissue mass
- The lesion permeates the marrow spaces
- Osteosarcomas usually infiltrate the marrow several centimeters away from the main tumor mass
- Skip lesions may be apparent that are separated from the main tumor by normal marrow
- Osteosarcomas may also have cartilaginous components that appear as translucent lobules, and/or fibrous components that are tan, soft to firm rubbery areas
- Osteoblastic areas are usually white to yellow, firm, hard and gritty
- The consistency of the tumor depends on the amount of osteoid deposition, cartilaginous and fibrous areas
- Foci of hemorrhage and necrosis are common
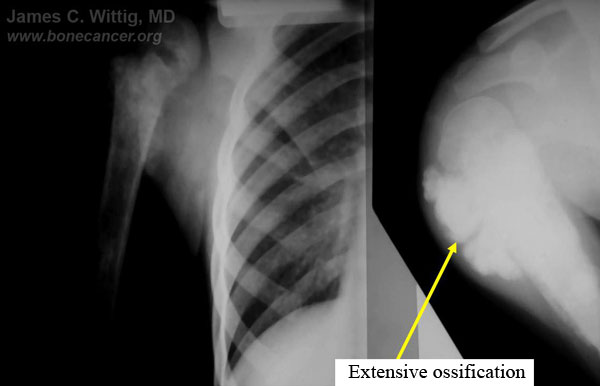
- Periosteal reactions such as the Codman's triangle are apparent at periphery of soft tissue mass
- Osteosarcomas rarely penetrate the growth plate grossly
- Invasion of the joint is uncommon but can occur by cortical penetration, joint capsule extension, or extension along cruciate ligaments
| Roll over the images for more information |
 |
 |
 |
 |
| Roll over the images for more information |
 |
 |
 |
 |
| Roll over the images for more information |
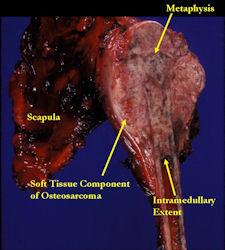 |
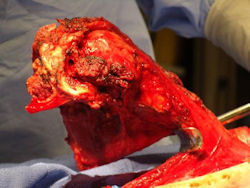
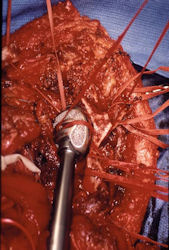
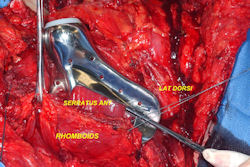
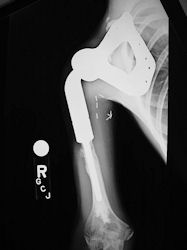
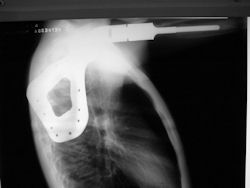
- originates from metaphysis of the proximal humerus, extends into surrounding soft tissues
- large soft tissue component that is crossing the glenohumeral joint
- was removed via an extra-articular resection, including scapula (Tikhoff-Linberg resection)
Microscopic Pathology: Conventional Osteosarcoma
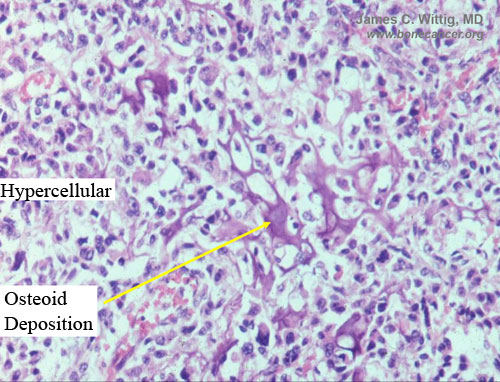
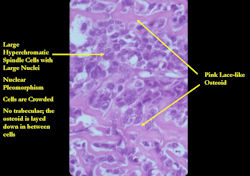
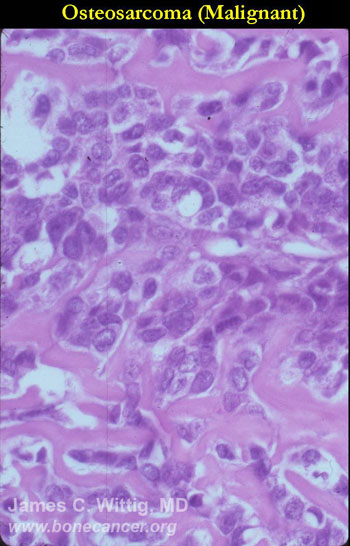
- High grade anaplastic tumor
- Hypercellular, spindle cell tumor with extensive pleomorphism (cells are different sizes and shapes)
- Large nuclei and little cytoplasm (high nuclear to cytoplasmic ratio)
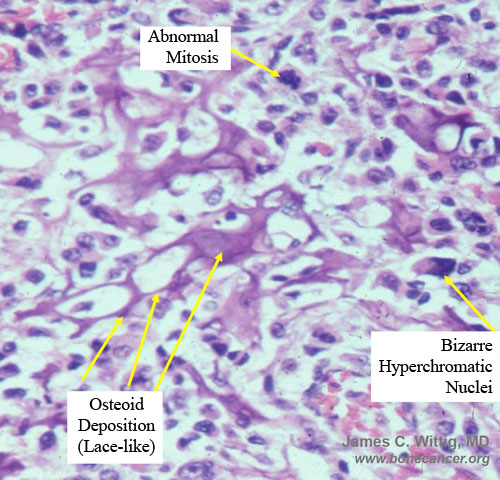
- Many mitotic figures and abnormal mitoses
- Bizarre appearing cells with hyperchromatic nuclei
- Osteoid production
- Osteoid is often laid down in lace-like pattern in between the malignant cells
- Osteoid stains pink to red with H&E stains
- No osteoblastic rimming (the cells do not line up on the surface of a trabecula of osteoid as in osteoblastoma or osteoid osteoma)
- Osteoid may or may not mineralize; degree of mineralization determines how visible it is on x-ray
- May have other elements such as cartilage, fibrous tissue, small round blue cells, giant cells and telangiectatic changes
| Roll over the images for more information |
 |
 |
 |
- Fibrosarcoma subtype of conventional osteosarcoma may show spindle cells that arrange themselves in a "herringbone" pattern
- Malignant fibrous histiocytoma (MFH) subtype of conventional osteosarcoma often demonstrates a "storiform" arrangement of cells. There are pleomorphic spindle cells mixed with large, bizarre cells.-
- Giant cell rich conventional osteosarcomas may have large sheets of reactive giant cells that may obscure the underlying sarcoma. Osteoid production may be sparse.
- Preoperative chemotherapy changes the microscopic appearance of conventional osteosarcomas. Necrotic osteoblastic foci appear as acellular osteoid matrix. The cells are killed by the chemotherapy, but the osteoid remains. Fibrosarcomatous areas are replaced by collagen and scar tissue, granulation tissue and inflammatory cells. Chondroblastic foci will have "ghost cells" in lacunae (acellular chondroid tissue)
Differential Diagnosis (Pathologic Differential):
- Osteoblastoma
- Osteoid Osteoma
- Fracture Callus
- Giant Cell Tumor
- "Dedifferentiated" Chondrosarcoma
- Malignant Fibrous Histiocytoma (MFH)
- Fibrosarcoma of Bone
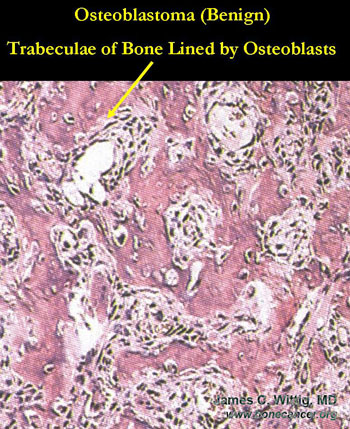
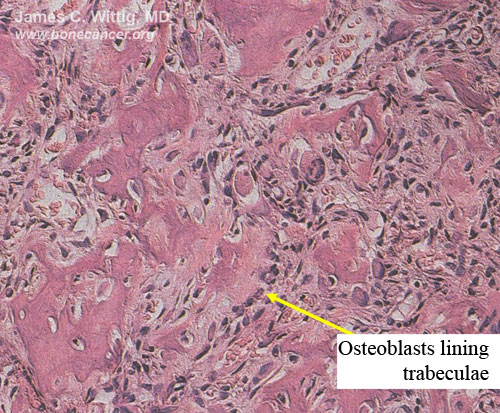
Osteoblastoma (benign bone-forming tumor) vs. Osteosarcoma
- Osteoblastoma usually geographic, benign appearing lesion on x-ray
- Osteosarcoma usually permeative on x-ray
- Osteoblastomas have thick, irregular trabeculae of osteoid and woven bone with osteoblastic rimming
- Trabeculae of osteoblastoma are separated by intervening stroma with capillaries and osteoclasts/giant cells
- Osteosarcomas infiltrate surrounding lamellar bone, whereas osteoblastomas grow with a pushing margin. There is a sharp cut off between the osteoblastoma and normal bone at the periphery of the osteoblastoma. Osteoblastomas do not permeate the surrounding lamellar bone.
- There is no cartilage in an osteoblastoma, unless it has fractured the bone or the lesion has been biopsied
| Roll over the images for more information |
 |
 |
| Roll over the images for more information |
 |
"Dedifferentiated" Chondrosarcoma vs. Chondroblastic osteosarcoma
- Dedifferentiated chondrosarcoma consists of a low grade cartilaginous component with a high grade anaplastic osteoid-producing component. The high grade anaplastic component is sharply cut off from the low grade cartilaginous component
- Chondroblastic osteosarcoma contains high grade cartilage mixed with osseous component
- The x-ray of a dedifferentiated chondrosarcoma shows a separate distinct cartilaginous component
Treatment
Treatment is often dictated by National Children’s Oncology Group Protocols (COG Protocols):
- 1.) Preoperative (induction or neoadjuvant) chemotherapy (typically 2-3 cycles, then surgery):
- Adriamycin (doxorubicin)
- Cisplatinum (cisplatin)
- High Dose Methotrexate (HDMTX)
- Ifosfamide/Etoposide in some regimens
- 2.) Surgery:
- Wide surgical resection/limb salvage (most extremity lesions can usually be treated with limb salvage when detected early)
- Amputation (5% of extremity lesions)
- 3.) Postoperative (adjuvant) chemotherapy:
- Same regimen as preop; usually 4 cycles
Preoperative (Neoadjuvant) Chemotherapy
- Preoperative chemotherapy essentially kills the primary tumor and makes it easier to perform a limb sparing surgery instead of an amputation. At the same time, the chemotherapy kills microscopic cells that may have traveled to the lungs
- Preoperative chemotherapy is one of the main reasons limb-sparing surgery can be performed in the majority of patients with an osteosarcoma
- The tumor may not actually shrink much in size because of the presence of osteoid; however, when the cells of the tumor are killed, the body begins to heal against the tumor. Normal tissue is more clearly delineated during surgery from the tumor and abnormal tissue
- Radiological studies may be useful in determining if the osteosarcoma has had a good response to chemotherapy preoperatively:
- X-rays and CT scans may show that intense ossification of the tumor occurs after a good response to preoperative chemotherapy
- The CT scan will often show a peripheral zone of calcification (egg shell around the tumor) when the tumor has had a good response
- MRI is not useful for evaluating response of osteosarcomas to preoperative chemotherapy
- Bone scan and/or thallium scan may be useful. Decreased uptake after preoperative chemotherapy compared to the uptake on initial diagnosis may be indicative of a good response
- Formal angiograms where dye is injected into the blood vessels of the extremity demonstrate the vascularity of the tumor. Viable tumors have significant blood vessels and therefore fill with the dye, causing a tumor blush. If an osteosarcoma has had a good response to chemotherapy, the vascularity will disappear and there will be little to no tumor blush. Angiogram is the gold standard for evaluating response to preoperative chemotherapy; however, it is not routinely performed because it is invasive.Estimating response before the surgical procedure may help with surgical planning and may also help in deciding whether a limb sparing surgery or an amputation should be performed. It is often not necessary to remove as much surrounding normal tissue around an osteosarcoma when the tumor has demonstrated a good response, as opposed to those tumors that do not demonstrate a good response.
- The final estimate of response to preoperative chemotherapy occurs when the specimen is surgically removed and analyzed by the pathologist. This estimate helps predict prognosis. A "good response" (usually greater than 90% of the tumor killed) has been correlated with approximately a 90% cure rate.
| Roll over the images for more information |
 |
Surgery
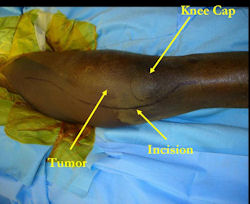
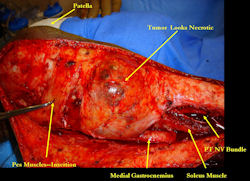
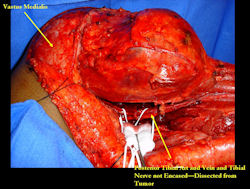
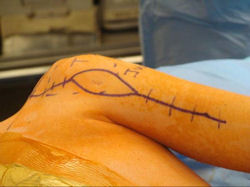
Examples of radical limb sparing surgeries for osteosarcomas in various anatomic locations (distal femur, proximal tibia, proximal humerus, scapula)
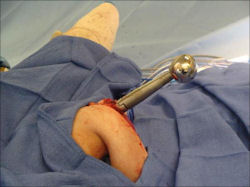
In each case, the tumor and bone from which it arose were resected. This required meticulous dissection, mobilization and preservation of adjacent pertinent neurovascular structures. In each case presented here, the defect was reconstructed with a special modular segmental tumor prosthesis. This also replaces the adjacent joint in many instances.
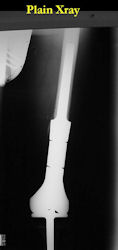
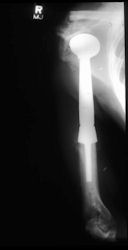
Limb-sparing surgery for osteosarcoma of distal femur - Plain Xrays
|
 |
 |
Limb-sparing surgery for osteosarcoma of distal femur - Intraoperative photos
|
 |
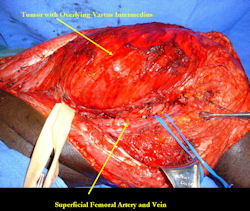 |
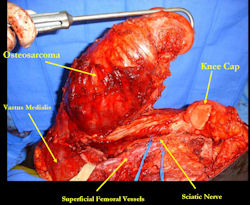 |
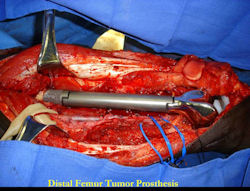 |
Limb-sparing surgery for osteosarcoma of distal femur - Outcome after surgery
|
 |
 |
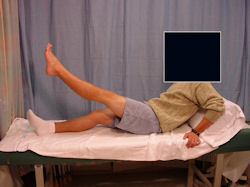 |
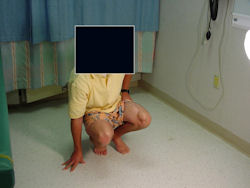 |
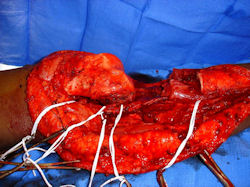
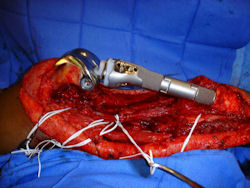
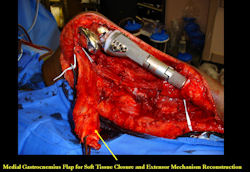
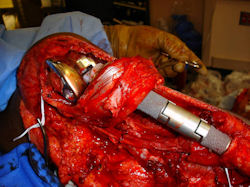
Proximal Tibia Osteosarcoma: Limb-Sparing Surgery
|
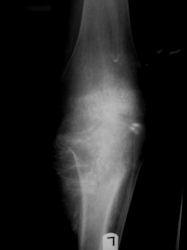 |
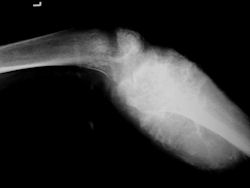 |
Intraoperative photos
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
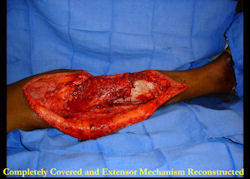 |
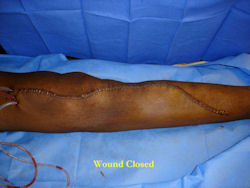 |
Postoperative X-Rays
|
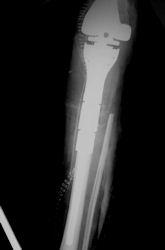 |
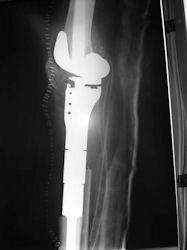 |
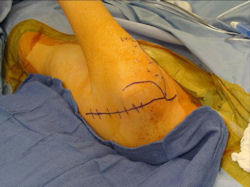
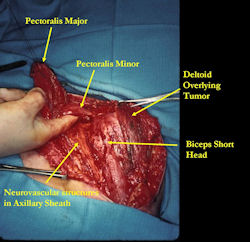
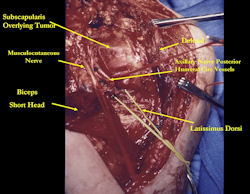
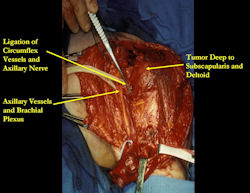
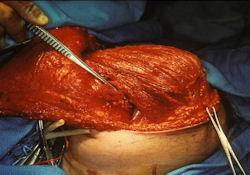
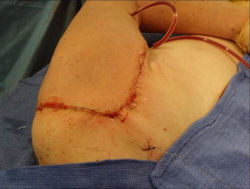
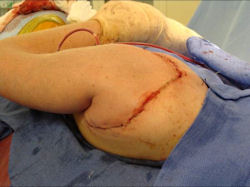
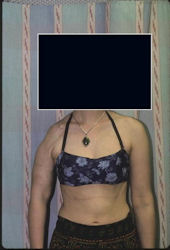
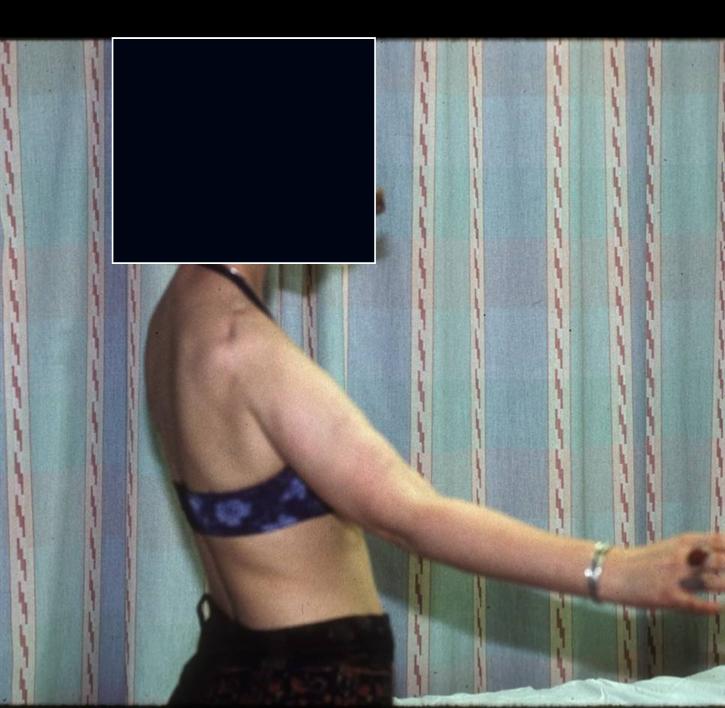
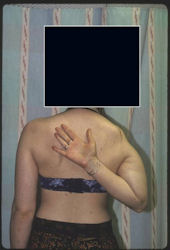
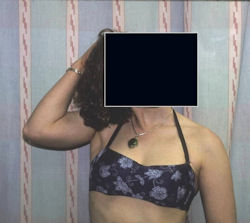
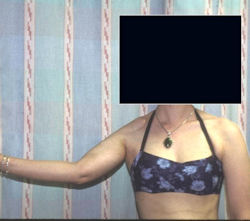
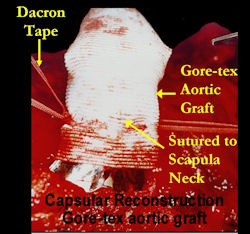
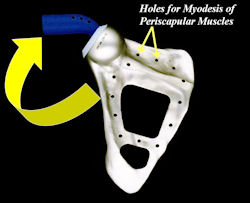
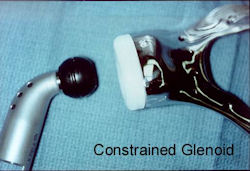
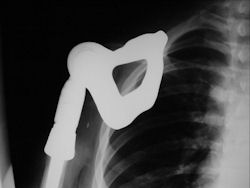
Proximal Humerus: Radical Limb Sparing Extra-Articular Resection and Prosethetic Reconstruction
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
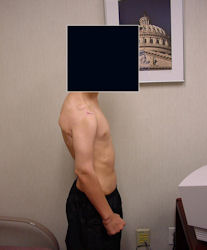 |
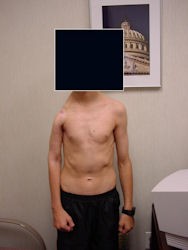
Results and risks associated with limb sparing surgery and prosthetic reconstructions
These types of surgeries are massive, risky, limb sparing surgeries that are performed instead of
- performing an amputation. The functional results after reconstruction with a tumor prosthesis should always be compared to what the results would be following alternative treatment, namely an amputation. The results never restore the function of the extremity to the same level as the contralateral normal extremity.
- The results shown here are particularly good results. While most patients achieve good results, not everybody achieves a good result because of various factors.
- Risks include infection; future loosening; prosthetic breakage and failure (prosthesis can wear out in the future); stiff joints; joint weakness; limb length inequality; tumor recurrence; persistent pain, weakness or stiffness in the arm or leg or adjacent joint; nerve or vascular dysfunction/injury; the possibility that complications could require additional surgeries for treatment and even an amputation for treatment.
- Patients are advised not to perform activities that require running or jumping, as well as activities that place the patient at a significant risk of falling or breaking the prosthesis or bone that the prosthesis is anchored in.Patients with localized, nonmetastatic osteosarcoma at presentation who are treated with surgery and chemotherapy have cure rates of 70% to 80%.
- If there has been a "good response" to preoperative chemotherapy as determined by analyzing the removed specimen, then there is approximately a 90% chance of being cured
- There is no difference in survival rates whether a limb sparing procedure or an amputation is performed
- Patients who present with or develop metastases to the lungs have a worse prognosis although may be capable of being cured if the pulmonary metastases can be resected.
- Patients with bone metastases have a very poor prognosis
- Skip metastases confer a poor prognosis
- Changing the chemotherapy regimen postoperatively for patients who did not have a "good response" has not been shown to change prognosis as of 2008
